Implantation damage controls most of the dopant diffusion effects seen in modern silicon technologies. Despite more than 20 years of effort, understanding of defect-coupled dopant diffusion still falls short of what is practically required to support state-of-the-art silicon technology development. To obtain models that are as predictive and efficient as possible, we must combine the best of our physical understanding with measurements of dopant profiles for technology-relevant conditions. This paper presents experimental results that provide insight into damage generation and annealing processes and discusses practical modeling approaches to support technology development.
Gains in performance and circuit density from ever smaller transistors motivate the continuing development of silicon IC technology. As transistor channel lengths decrease, the vertical and lateral dopant distributions in the source/drain regions must also scale to maintain acceptable device behavior. The majority of diffusion seen in most current process technologies is transient enhanced diffusion (TED) caused by damage generated during ion implantation. Disruption of the crystal lattice by collisions with the implanted ions also reduces channeling, modifying the dopant distribution for subsequent implants. Understanding the interactions between implantation and diffusion is central to submicron transistor technology.
Figure 1 shows dopant distributions in the source/drain region of a MOS transistor. During the tip and source/drain implants, ion collisions knock silicon atoms from their lattice sites, leaving a vacant lattice site behind and sending a new energetic silicon atom into the surrounding lattice. Secondary recoils generated by this atom create a cascade of displaced atoms of decreasing energy. Eventually the recoil atoms do not have enough energy to cause further displacements and come to rest as silicon self-interstitials. Some of the vacancy and interstitial point defects created by this process may recombine locally, but others remain as defect clusters. These dissolve during high-temperature annealing, releasing mobile point defects that control the diffusion of the implanted
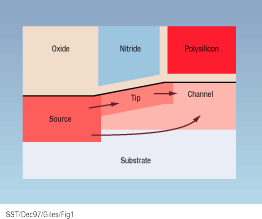
Figure 1. Implantation damage from the source and tip regions control transient enhanced diffusion throughout the structure.
ions as well as any other dopants that might be present. Since the point defect concentrations released are several orders of magnitude higher than the thermal equilibrium defect concentrations, dopant diffusivities also increase by several orders of magnitude. Eventually, recombination and trapping remove the point defects, and the dopant diffusivities return to normal. This phenomenon, TED, dominates dopant diffusion.
Since point defects diffuse rapidly, they can move from the implanted region into the channel, leading to additional diffusion there. Defect gradients can also drive channel dopant toward the surface, modifying the threshold voltage of the device [1]. The surface can act as a strong sink for dopant, modifying both the vertical and lateral diffusion. Amorphizing implants introduces further complications: solid phase epitaxial regrowth and the possible formation of a dislocation loop layer. The final doping profile results from the interactions among multiple dopant types and multiple implant damage generation and annealing cycles.
Implant damage creation
Implant damage creation is a complicated process involving the generation and recombination of recoil cascades and the formation of point and extended defects and dopant-defect clusters. Physically based modeling of this process is an active area of research, and is being addressed with a hierarchy of modeling approaches. Developing and validating these models is difficult; direct measurement of defect types and distributions is not possible in most cases. Significant progress has been made in recent years, using TEM to observe the time evolution of {311} and other extended defects [2], but these techniques provide information about the silicon after significant annealing, not after ion implantation alone, mixing implantation and diffusion effects.
Despite the complexity of the physical processes, the simplistic "+1" model [3] is surprisingly useful in providing initial conditions for point defect-based diffusion models. Figure 2 schematically shows recoil generation during ion implantation and recombination during the early stages of annealing. Although each implanted ion generates many interstitial-vacancy (Frenkel) pairs, bulk recombination removes the majority of them. Annealing also moves the implanted dopant atoms onto silicon lattice sites, displacing a single interstitial for each implanted ion. These "+1" interstitials have no corresponding vacancy with which to recombine, so they survive and influence dopant diffusion for much longer times.

Figure 2. Simplified model of damage generation and annealing resulting in the "+1" interstitial profile.
The model avoids detailed modeling of the implant damage process by making the starting interstitial profile the same as the implanted dopant profile. A scaling factor for the interstitial dose is often used as a fitting parameter, at least in part due to uncertainties in the other parameters of the diffusing system. Experimental measurements of interstitial dose trapped in {311} defects in the early stages of annealing support a scaling factor close to 1.0 [4]. The success of this approach implies that interstitial-vacancy recombination occurs rapidly, and the initial number of Frenkel pairs generated during implantation does not dominate TED.
Experimental validation of the "+1" model. The silicon surface is a very important recombination site during TED because it can act as an independent sink for both interstitials and vacancies. Distance from the surface determines how far defects must diffuse before being recombined [5] and strongly affects the annealing rate for damage. According to the "+1" model, the effective initial interstitial distribution (and so, TED) for two implanted profiles with the same implanted dopant distribution but different total damage should be the same. This claim can be tested experimentally by comparing samples implanted at different energies and tilt angles such that the as-implanted vertical doping profile is the same but the total damage created by the implant is different. Tilted implants allow investigation of different energies without varying the distance of the dopant from the surface.
Our experiments used phosphorus implants into [100] silicon through a 100-? screen oxide at doses equivalent to 5 ? 1013 cm-2 normal to the surface. We chose energy-tilt combinations of 100 keV at 7?, 148 keV at 50?, and 181 keV at 60? to produce as-implanted profiles that were as similar as possible. Monte Carlo ion implantation calculations of Frenkel pair generation for these conditions predicted increased interstitials and vacancies with increasing implant energy (Fig. 3). The annealing conditions, 800?C for one hour in an inert ambient, were chosen to be sufficient to anneal out the implant damage completely.
Figure 4a shows SIMS profiles before and after annealing. The as-implanted profiles are indeed similar, with the higher-energy implants slightly deeper and broader than the 100 keV implant. Although normal phosphorus diffusion is negligible under these annealing conditions, TED causes significant diffusion. To first order, the implants experience the same amount of TED and produce the same final phosphorus profile, validating the "+1" model assumption. On closer examination, the profile motion decreases slightly with increasing implant energy. Assuming a constant effective diffusivity, the diffusion lengths are 69, 65, and 60 nm for the 100, 148, and 181 keV cases, respectively. This difference may be due to a sputtering-induced increase in the net loss of interstitials for the higher implant tilt angles. Lower implantation energies of 30 keV (7?) and 62 keV (60?) produce the same behavior, coupled with a strong segregation of phosphorus to the silicon/oxide interface (Fig. 4b).
Implantation channeling profile measurements. When ions are implanted into a crystalline lattice in channeling directions, a certain fraction will travel large distances into the lattice with few nuclear collisions. These channeling ions produce a characteristic tail in the dopant profile. If the lattice is damaged, the probability of traveling large distances without nuclear interactions is strongly reduced. Therefore channeling can serve as a sensitive measure of the amount of damage present in the substrate [6]. Figure 5 shows how the channeling profile measurement (CPM) technique is applied. A 200 keV silicon implant with a dose of 1014 cm-2 and a beam current of 1 mA creates a damage profile. An annealing cycle at a particular time and temperature removes some of the damage from the wafer. Then, a boron channeling profile implant is made at 140 keV, 0? tilt, with a dose of 1013 cm-2. This implant creates a deep channeling tail for an undamaged wafer. Boron profiles for different damage annealing conditions illustrate the remaining damage. The technique is sensitive to silicon damage doses as low as 1013 cm-2, so small differences in remaining damage from higher dose implants can be detected.
The "+1" model requires that most of the Frenkel pairs generated in the collision cascades rapidly recombine during the earliest stages of the anneal, while the wafer temperature is ramping up to the target anneal conditions. Figure 6 shows the effect of annealing the silicon damage for 5 sec and 20 sec at 600?C. Even at this low temperature, a large recovery of the implant damage occurs within 5 sec, leaving some residual damage that appears to be stable at 600?C. The very rapid recombination of the initial damage cascade implies an extremely small energy barrier to interstitial-vacancy recombination.
CPM can also measure the stability of the residual damage at higher annealing temperatures. Figure 7 compares anneals for 10 sec at 600 and 900?C, showing a second stage of damage recovery. The 900?C boron profile is very close to the undamaged case. However, the observation of TED over a few minutes at these temperatures means that excess interstitials must still be present in some form. The staged nature of damage recovery can lead to multiple bursts of TED at different anneal temperatures, as discussed below.
Transient enhanced diffusion
Understanding of the physical mechanisms controlling TED has made great progress in the past few years by building on a basic understanding of defect behavior and dopant-defect interactions [7, 8]. Experimental evidence has verified the strong correlation among {311} defects, dopant-interstitial clusters, and TED. Physical models with different levels of complexity have been shown to give good agreement with experiment, but comparisons are usually limited to a small number of implant/anneal combinations. While this approach is entirely appropriate for the development of physical understanding, modeling for technology development must account for all the implantation and annealing steps in a technology flow. This section examines some of the challenges of modeling for technology development, focusing on conditions typical of the tip region doping in a MOS device.
TED across multiple anneals. Annealing steps include both the deliberate high temperature anneals designed to drive in and activate dopant, and the incidental, lower-temperature anneals associated with the deposition of some material layers. At lower temperatures, repairing implantation damage takes longer, so TED extends for a longer time. This effect can increase total dopant diffusion as annealing temperatures are reduced: the lower-temperature steps dominate the final dopant profile. The low energies and high doses used in modern technologies have more complex interactions than this simple view would suggest, making it harder to predict the outcome of a multitemperature anneal sequence.
The implant was made at 5 keV and 0? tilt into bare silicon at a dose of 1015 cm-2. The profiles display several interesting characteristics. First, the peak shows a displacement toward the surface and a decrease in dose during the first annealing step. This effect is discussed in the next section. Second, TED at 650 and 750?C appears to be complete after 90 min, but a subsequent RTA step causes a second burst of TED and additional profile motion. The second TED phase is complete in <10 href="javascript:OpenLargeWindow(265014,650,666,">
Relating these results with the CPM results described earlier, the implantation damage appears to form defects with several different annealing temperature thresholds. Annealing at an intermediate temperature produces a burst of TED as some categories of defects dissolve, but other defects may cause a further burst of diffusion when a higher-temperature anneal occurs. For high-concentration arsenic implants, arsenic-vacancy cluster formation may inject additional defects [9]. These clusters form during deactivation from the highly activated state left by epitaxial regrowth of the amorphized surface layer.
Dopant redistribution and solubility effects. The silicon/oxide interface is a strong sink for dopants as well as point defects. When trapped in the oxide, the dopant is electrically inactive [10] and is removed when the oxide is etched away. This behavior, observed for phosphorus, boron, and arsenic, can result in the loss of half or more of the implanted dopant. The effect is clearly visible in Fig. 4b, which shows SIMS profiles for annealed phosphorus before and after the surface oxide was removed using HF. The profile after etching contains only 50% of the original implanted dose. Surface loss can also significantly reduce lateral dopant motion near the surface at a mask edge, a critical region for technology applications.
The dose loss effect is linked to TED because the large transient diffusivity allows rapid transport of dopant to the interface. After amorphizing implants, solid phase epitaxial (SPE) regrowth may induce a partial sweeping out of dopant from the regrown layer. For example, in Fig. 8, the lowest-temperature anneal shifts the arsenic peak toward the surface and reduces the total arsenic dose. Since SPE occurs rapidly at temperatures above 500?C, we expect it to be completed during the temperature ramp-up period as wafers are pushed into the furnace.
For boron, an additional important effect is reduction in effective boron solubility during the transient [11], due to the formation of boron-interstitial clusters [12]. Figure 9 shows experimental measurements for annealing of BF2 and B implants. The BF2 example was implanted through a 130? oxide at 45 keV to a dose of 2.5 ? 1014 cm-2 and annealed at 900?C. The B example was implanted at 40 keV through the same oxide to a dose of 2 ? 1015 cm-2 and annealed at 800?C. For short anneal times, TED is in progress and the profiles show a characteristic kink near the intrinsic carrier concentration [13].
Modeling for technology development
Early dopant diffusion modeling in silicon was based on work [14] that interpreted the Fermi level dependence of diffusivity as a consequence of the equilibrium behavior of charged point defects. To extend this interpretation to the far-from-equilibrium conditions induced by ion implantation, a model must include the creation and evolution, in both time and space, of the relevant defect types and their interaction with free dopants and defects. The most straightforward approach, the so-called fully coupled approach, is to solve the continuity equations for the various defect types while also solving dopant diffusion. This approach is simple, direct, physical, and has the greatest possibility of being predictive across a wide range of process conditions. The three major drawbacks of the fully coupled approach are: lack of well-accepted physical mechanisms spanning the full range of conditions of technological interest for both implant damage creation and dopant-defect interaction; computational cost; and the difficulty of calibrating a complex and strongly coupled model system.
Given the limitations and costs of the fully coupled system and the need for calibrated models that keep pace with technology development, we have decided to use a hierarchical approach. Exploration of defect physics and evaluation of new defect models requires solutions of the coupled system. For day-to-day technology development, however, the best approach uses the one dopant diffusion equation system, obtaining an effective dopant diffusivity from a model for implant damage annealing in time and space. A semi-empirical implant species/energy/dose dependent damage creation/accumulation model provides the initial conditions.
Our effective diffusivity model represents implant damage by a dimensionless quantity representing an effective damage dose. Analytic distribution functions incorporate spatial effects, allowing, for example, dopant up-hill motion driven by the defect gradient near the surface. The effective damage dose decreases during annealing with limited amounts of the total damage available for repair at lower annealing temperatures. The dopant diffusivities are greatly enhanced while the damage clusters are dissolving and sharply drop back to the intrinsic values when the damage is annealed out (Fig. 10). While a clear physical picture is still needed to develop useful forms for the damage annealing models, the practical dependence of the simulation on details of the underlying physical mechanism is much reduced. When technology development demands capability in a brand-new application area or revolutionary progress has been made in physical understanding, the effective diffusivity model offers greater flexibility and extensibility, as well as a much shorter turn-around time for modification and calibration. Figures 11 and 12 show example effective diffusivity calculations based on experimental results presented earlier. We include dose trapping and re-emission from the silicon/oxide interface [15], and the effect of dopant redistribution during SPE. Good agreement can be found for simulations of complex implant/anneal sequences of technological interest.
Asignatura: EES





















